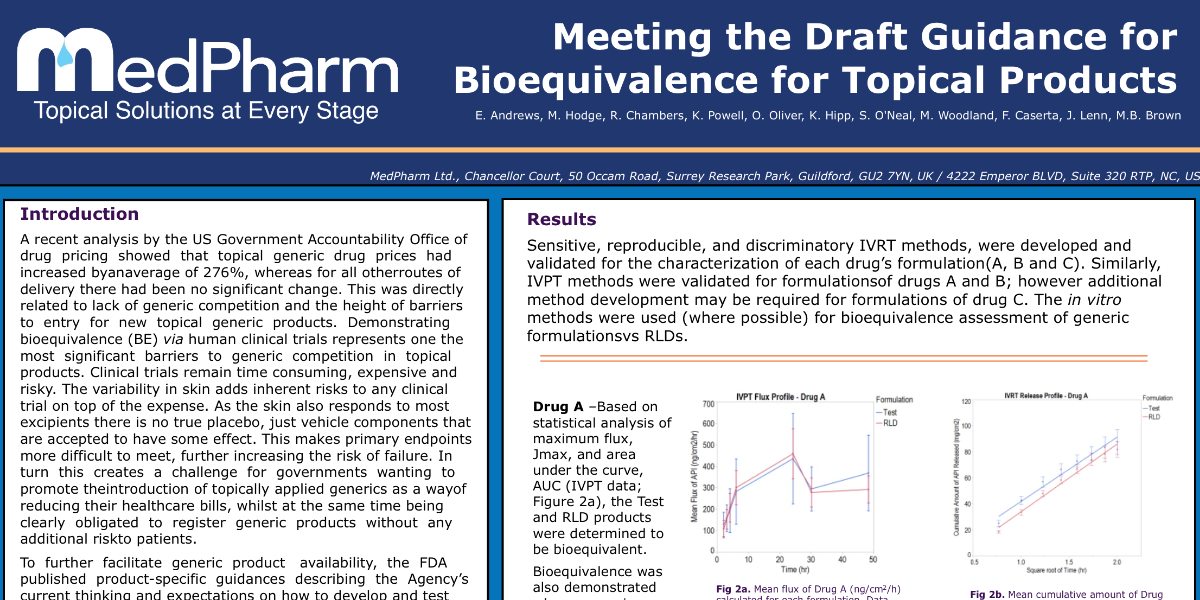
Meeting the Draft Guidance for Topical Products
Demonstrating the Skin Penetration Potential of a Large Aptamer
Challenging Skin Penetration Assumptions with GSK and the University of Reading
New research is redefining assumptions about the skin penetration potential of large molecular weight drugs, offering hope for new therapies in difficult-to-treat dermatological diseases.
It has long been believed that molecules above 1,000 Daltons cannot penetrate the skin at therapeutic levels, limiting their use in topical drug delivery. However, researchers from GSK and the University of Reading collaborated with MedPharm to challenge this paradigm.
For this study, MedPharm developed targeted disease activity skin models to test the ability of a 22k Dalton aptamer—a specialized RNA molecule under increasing pharmaceutical interest—to penetrate the skin and demonstrate therapeutic efficacy.
The results revealed that the aptamer not only penetrated the skin barrier but also showed measurable therapeutic activity. These groundbreaking findings were published in the Journal of Investigative Dermatology and suggest that the traditional molecular weight limitations for topical drug delivery may be more flexible than previously thought.
Proving Pharynx Formulation Equivalence with Specific Models
Reckitt Benckiser scientists came to MedPharm with a challenge: could we devise models to determine how well their flurbiprofen throat spray and lozenge deliver the drug into human pharynx tissue?
In response, MedPharm’s team developed clinically relevant in vitro models and incorporated them into a comparative study evaluating the permeation of each flurbiprofen formulation. The study utilized ex vivo pharynx tissue from pigs for model development, and human cadaver tissue for final experimental validation.
MedPharm’s findings demonstrated that flurbiprofen permeation was equivalent across both delivery forms. These data also enabled the calculation of a clinically relevant dose for this non-steroidal anti-inflammatory drug.
The results were presented at the 3rd German Pharm-Tox Summit in 2018 and used to educate key opinion leaders on the demonstrated equivalence of Reckitt Benckiser’s formulations.
Cost-Effective Process Development Delivers Critical Clinical Trial Supply
MedPharm was asked to provide multiple 25 kg batches of a complex cream for a Phase 2 atopic dermatitis clinical trial on behalf of a global pharmaceutical company. A failure to supply or delay would have carried significant financial consequences for the project. The client specifically valued MedPharm’s detailed understanding of the product’s multi-stage manufacturing process.
Our experts leveraged their deep knowledge of formulation and years of experience scaling complex semi-solid products to identify the Critical Process Parameters (CPPs): homogenization speed and time, and cooling rates at various stages. A factorial design of 12 runs was created using 1 kg lab reactors to model the commercial scale.
The key Critical Quality Attribute (CQA) was the rheology profile of the final product. This work revealed the product’s rheology was particularly sensitive to homogenization time and speed, enabling our team to set precise processing parameters. A successful initial run at scale confirmed the robustness of the selected approach.
MedPharm subsequently produced over 15 clinical batches with consistent, reproducible quality using this optimized process. Without this early process evaluation, the client likely would have faced significant material loss and delays. Our targeted approach ensured efficient use of a high-value API and helped keep the clinical trial on track.
Article: Establishing a Rigorous and Robust Approach to Process Development
The aim of process development is to establish optimal process parameters and conditions for future large-scale batches or commercialization, ensuring that the product can be successfully manufactured in compliance with required quality and regulatory standards. This ultimately means that an in-depth understanding of the formulation is key.
In a recent two-part article with Manufacturing Chemist, Joseph Holt, Head of Process Development at MedPharm, explores the challenges facing developers when scaling-up processes for semisolid drug product production and highlights the additional difficulties associated with generic drug development and manufacturing.
Article: Excipient Selection in Topical Process Development
Unlike solid dosage drug products and sterile injectables, topical and transdermal formulations often contain a higher number of excipients, making them more complex to develop and scale. Given that the processes that can be used for manufacturing a drug product are largely impacted by the specific components of that product’s composition, a strong comprehension of the formulation is key.
Throughout drug product development, it is critical to understand the excipients, processing parameters, and quality attributes specific to the product so that optimal excipients and manufacturing methods can be established for future commercialization.
Roundtable: Increasing the Robustness of IVPT Experiments
In a recent Analytical Special Feature with Drug Development & Delivery, industry experts weigh in about the the impacts of AI and automation in analytical labs.
MedPharm CSO, Dr. Jon Lenn, discusses our unique automated diffusion cell system, MedFlux-HT®, and transepidermal water loss (TEWL) instrument used for IVPT studies.
Learn more about the advantages of these systems in the lab, and read the full feature here.
Cost-effective process development of complex cream ensures consistent quality and on-time delivery of clinical trial material
MedPharm was asked to provide multiple 25 kg batches of a complex cream for an important Phase 2 atopic dermatitis clinical trial by a large global pharmaceutical company. Failure to supply or delay would have had major financial consequences for the project. Due to the nature of the request, the client valued MedPharm’s detailed understanding of the multi-stage manufacturing process.
Our experts used their understanding of the formulation and their many years of experience in scaling-up complex semi-solid products to identify the ‘Critical Process Parameters’ (CPP) as homogenization speed and time, and cooling rates at different stages. They laid out a suitable factorial design of 12 runs using 1kg lab reactors which model the large scale. The key ‘Critical Quality Attribute’ was identified to be the rheology profile of the final product. This work showed that the rheology could be particularly sensitive to the homogenization time and speed and appropriate parameters were set. An initial run at the larger scale confirmed that the process and parameters selected gave a product which met all specifications.
Subsequently, over 15 clinical batches were manufactured with reproducible quality using the optimized manufacturing process. Given the sensitivities to key parameters observed this almost certainly would not have happened without the process evaluation work. Additional large batch production would have wasted significant amounts of a high-value API and impacted the completion of the trial.
Teaming up with GSK and the University of Reading to demonstrate the skin penetration potential of a large aptamer
New research challenges current theories on skin penetration of large molecular weight drugs offering hope for the discovery of new treatments of difficult-to-treat dermatological diseases.
It is generally agreed that drugs with molecular weights above 1000 Daltons did not penetrate the skin at therapeutic levels and so weren’t applied topically for the treatment of dermatological diseases. However, researchers from GSK and the University of Reading teamed up with MedPharm to challenge this current theory. MedPharm created specific disease activity skin models for a study designed to test the penetration of a 22k Dalton aptamer, which is a type of RNA molecule receiving notable interest for its potential pharmaceutical activity.
The results revealed this aptamer both penetrated the skin and showed therapeutic activity. The findings were subsequently published in the Journal of Investigative Dermatology and suggest the boundaries of molecules suitable for topical treatments can be expanded. This offers hope for the discovery of new treatments of difficult-to-treat dermatological diseases.